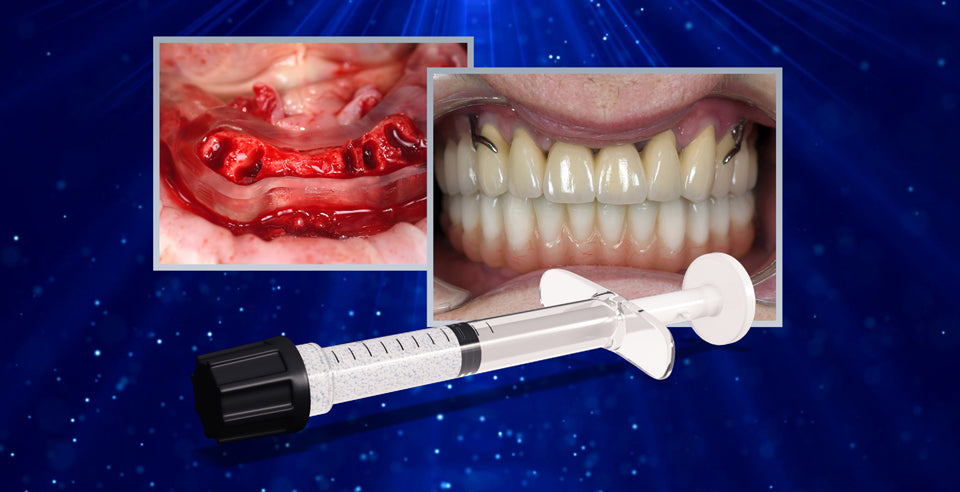
DSI Alyoss Plus is an allograft material made up of cortical and cancellous bone. The material is packaged in a ready-to-use syringe, making for a quick and easy delivery method. The bone is extracted from sections of the illium and ground into a variety of particle sizes, resulting in a one-of-a-kind blend. DSI Alyoss Plus, as a cortico-cancellous allograft, provides excellent structural support to the site of treatment while inducing rapid new bone formation. The cancellous bone's porous architecture acts as a scaffold, encouraging the ingrowth of bone vessels containing pre-osteoblastic and mesenchymal cells, promoting osteoconduction and accelerating revascularization. The cortical component contributes to the structural strength and support of the affected site due to its superior strength. DSI Alyoss Plus is a viable alternative to using a patient's autogenous graft, with a number of significant advantages. The material eliminates the need for a second surgical site, thereby eliminating the possibility of complications and infections, post-operative pain, additional anesthesia, and a longer healing process.
DSI Alyoss Plus is an excellent combination that provides this product with the ideal cell structure of cortical bone as well as an open scaffold and support provided by cancellous bone. All DSI Biomaterials are manufactured in accordance with stringent international standards. Numerous treatment sequences and exceptional measures ensure complete safety and high bone quality. Only EU-registered tissue banks are used as a source, and almost all donors are subjected to extensive screening and testing before being accepted into the banks. Following that, the bone is processed into DBM (Demineralized Bone Matrix) in highly innovative facilities using aseptic techniques to avoid cross-contamination and meet strict release standards. The final step in the manufacturing process is gamma sterilization, which results in a clinically effective product that is immune neutral.
The material's numerous advantageous properties make it ideal for sinus augmentation. The sinus floor can be more precisely adjusted with DSI Alyoss Plus, it is more biocompatible than other bone materials, and the healing process is faster and more predictable. The syringe ensures accurate and controlled placement for easier maxillary sinus filling during the sinus lift. DSI Alyoss Plus can be used alone or in combination with autogenous bone to improve the graft's osteoinductive properties.
Product SKU's
DSI Alyoss Plus, Syringe 0.25cc- AG25
DSI Alyoss Plus, Syringe 0.50cc- AG50
DSI Alyoss Plus, Syringe 1.0cc- AG100
Procedure for Handling
- Remove double or triple wrapping and using aseptic technique to avoid contamination. Check for any damages on the inner packaging
- Microbiological culture testing is recommended before the allograft tissue is soaked using saline solution
- Rehydrate the allograft at basin containing sterilized solution
- Rehydation of the allograft is recommended for more than 30 minutes
- The product should be cleansed for about 3 times by changing the solution to remove any remaining reagents
- All preparations should be carried out before transplanting the allograft
Features
- A corticocancellous mixture in a syringe
- Excellent clinical outcomes
- Excellent quality
- dependable and safe
- Extremely biocompatible
- There was no immunogenic reaction.
- Scaffolding for osteoconductive use
- New bone growth is accelerating.
- Handling that is both convenient and clean
- Time-saving and simple to use
- Only qualified donors from EU-registered tissue banks were used after extensive screening.
Benefits
- Cortical/Cancellous blend combines two of the most popular allograft particles used in dentistry, all in one syringe
- Allograft bone particulates supply a natural framework facilitating the attachment of osteogenic precursor cells.
- Safe alternative to an autograft with no need for a second surgical site.
- Used for volumetric enhancement, space maintenance and faster remodeling.
Use of Alyoss and Zenoss Matrix at lateral tooth replacement video process:
For more info,