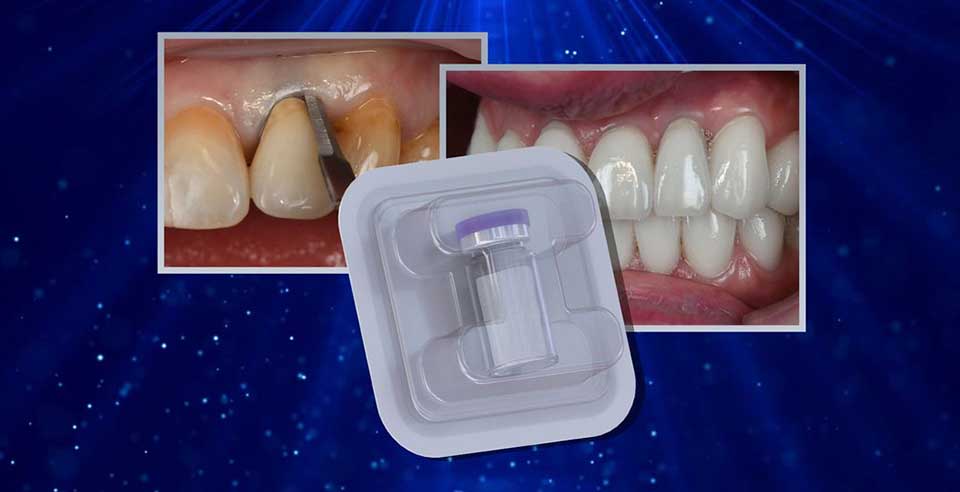
DSI Zenoss Matrix Bio+ is an innovative resorbable acellular collagen matrix that is ideal for a wide range of clinical cases and situations. To ensure the patient's complete safety, collagen is carefully purified during the manufacturing process to remove potential immunogens, bacteria, and viruses. The remarkable biocompatibility and superior mechanical properties of DSI Zenoss Matrix Bio+ make it an ideal material for soft tissue augmentation. DSI Zenoss Matrix Bio+ is an excellent alternative to autologous soft tissue grafts, allowing patients to avoid a number of disadvantages associated with autologous tissue harvesting, such as limited availability, second surgical site morbidity, and complications. Within 24 weeks, DSI Zenoss Matrix Bio+ has completely integrated with surrounding tissue. Its tridimensional architecture demonstrates exceptional revascularization cell proliferation abilities, which are enhanced by the interconnected structure. DSI Zenoss Matrix Bio+ is a safe and effective solution for repairing soft tissue deficiencies. DSI Zenoss Matrix Bio+ is also available as a plug, making it ideal for use in post extraction sites.
It can be used for a variety of purposes by ridge augmentation, extraction sites, restoration of congenital and acquired defects of bone and soft tissues, sinus-lifting, cystectomy, periodontitis, resection of the root apex.
Directions for use
- The general principles of sterile handling and patient medication must be followed when using the Zenoss Matrix.
- After opening, Zenoss Matrix is trimmed to the required size and shape with surgical scissors. Use of sterile metallic foil as a stencil is possible.
- The matrix should be used after wetting in sterile saline or the patient blood for 5 minutes, upon achieving needed flexibility. Size and shape should match closely match the defect.
- Due to the compact structure of this matrix, fixation or suturing is possible. After placement in covered sites, and the mucoperiosteal flap should be sutured over the matrix without tension. The wound should, whenever possible, be completely closed to avoid rapid resorption.
- Complete penetration of the Zenoss Matrix by blood allows close adaptation and adhesion of the matrix to the underlying surface. To avoid a bacterial contamination risk, minimize the contact of Zenoss Matrix with saliva or other media during the surgical stage.
- During the healing phase stress in the wound area from prosthetic pressure or palpation should be avoided. Intensive mechanical oral hygiene should be replaced by antibacterial rinsing (e.g., with chlorhexidine) for the first 3 weeks. Antibiotic therapy is prescribed at the discretion of the clinician
Product SKU's
| Zenoss Matrix Plug | 8 mm | DS-ZMP08 |
| Zenoss Matrix Plug | 12 mm | DS-ZMP12 |
| Small Matrix | 10x30 mm | DS-ZMM10 |
| Medium Matrix | 15x20 mm | DS-ZMM15 |
| Large Matrix | 20x30 mm | DS-ZMM20 |
Indications
- Gingival recession protection
- Increase in keratinized tissue
- Ridge augmentation of soft tissue
- Coverage of exposed roots
- After cyst removal, defects are filled.
- Extraction socket closure (socket seal)
- GBR/GTR soft tissue grafting technique
- As a blood clot stabilizer
Features
- Type I collagen that has been highly purified
- Secure and efficient
- Outstanding mechanical properties
- Excellent biocompatibility
- Vascularization occurs quickly.
- There is no need for autograft harvesting.
- In 3-6 months, the patient's own tissue will be completely integrated.
- Rapid hydration
- Handling is simple.
- During surgery, each piece can be easily trimmed for a perfect fit.
- Can be used on either side of the tissue or bone.
- Following a brief hydration period, it is terminally sterilized and ready for use.
Use of Alyoss and Zenoss Matrix at lateral tooth replacement video process:
For more information